O naší skupině
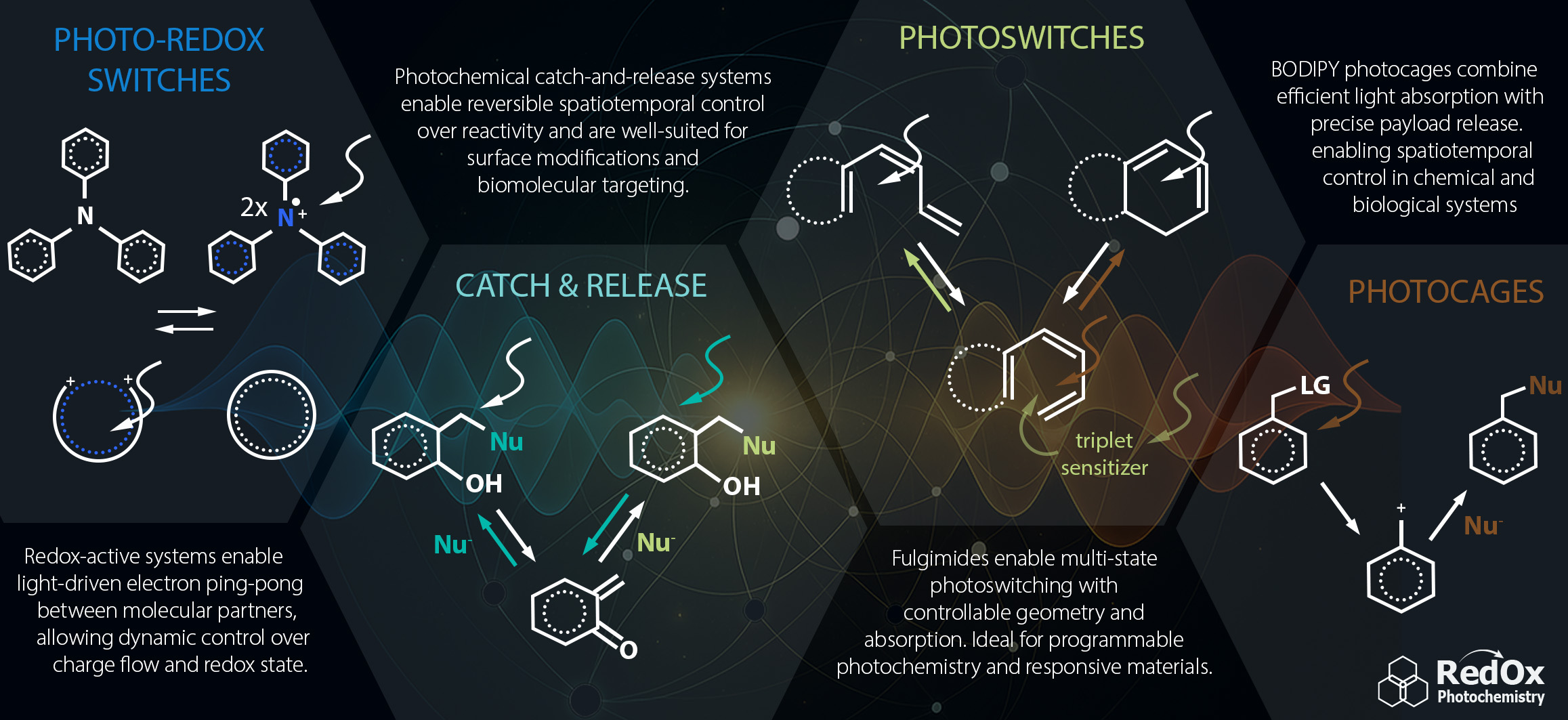
Výzkum v naší laboratoři je zaměřen na studium malých organických molekul, které podléhají přenosu elektronu a/nebo mohou být aktivovány světlem. Tato mezidisciplinární výzkumná oblast kombinuje organickou syntézu, elektrochemii, spektroskopii, fyzikální chemii a výzkum mechanismů procesů řízených světlem. Našimi hlavními cíli jsou: (i) schopnost přesné kontroly redoxních reakcí a přenosu elektronu v prostoru a čase, (ii) reversibilní přenos náboje mezi definovanými redoxními centry a (iii) vývoj metod pro stabilizaci organických radikálů a radikál iontů. Plánujeme použít tyto molekuly v různých aplikacích, jako jsou redoxní senzory, funkcionalizované povrchy, molekulární elektronika a „chytré“ materiály.